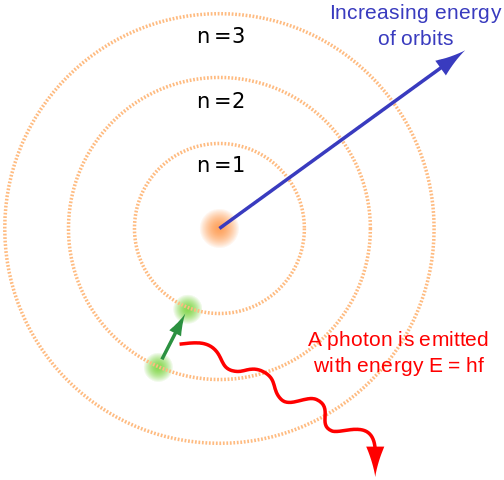
In general terms talking about the shape of orbits of an electron the number n determines the size and energy of the orbital for a given nucleus. The size of an atomic orbital is associated with Select one.

Orbital Chemistry And Physics Britannica
The spin quantum number ms.

. N the principal quantum number describes the energy of the electron. The principle quantum number n. The magnetic quantum number is concerned with the orbitals orientation in space.
The magnetic quantum number m. The size of the atomic orbital is associated with. The orbitals shape is explained by the angular quantum number.
B the angular momentum quantum number l. The angular momentum quantum number l the. Governed by the magnetic quantum number ml.
Spin quantum numberms E. B the angular momentum quantum number l. This function can be used to calculate the probability of finding any electron of an atom in any specific region around the atoms nucleusThe term atomic orbital may also refer to the physical region or space where.
The size of an atomic orbital is associated with the principal quantum number n. C the magnetic quantum number ml. The magnetic quantum numberml D.
As n increases the orbital size also increases. Governed by the angular momentum quantum number l orientation in space. C the magnetic quantum number ml.
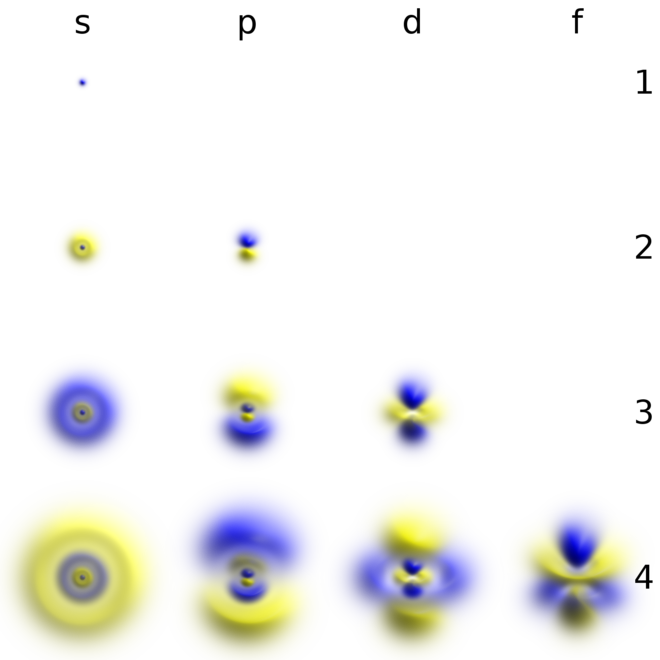
Size of the orbital can not be explained by any quantum number O e. These properties depend on the values assigned as the quantum numbers. Atomic orbitals are associated with characteristic Atomic orbitals are associated with characteristic energies sizes shapes and orientations in space.
The angular momentum quantum number l b. The properties of the atomic orbital are actually dependent on the quantum numbers. The magnetic quantum number ml c.
Governed by the principal quantum number n shape of atomic orbital. The angular momentum and magnetic quantum numbers together e. Angular momentum and magnetic quantum number together.
Principal Quantum number n b. Chemistry questions and answers. The angular momentum quantum number.
E the angular momentum and magnetic quantum numbers together. The size of an atomic orbital is associated with _____. The spin quantum number ms d.
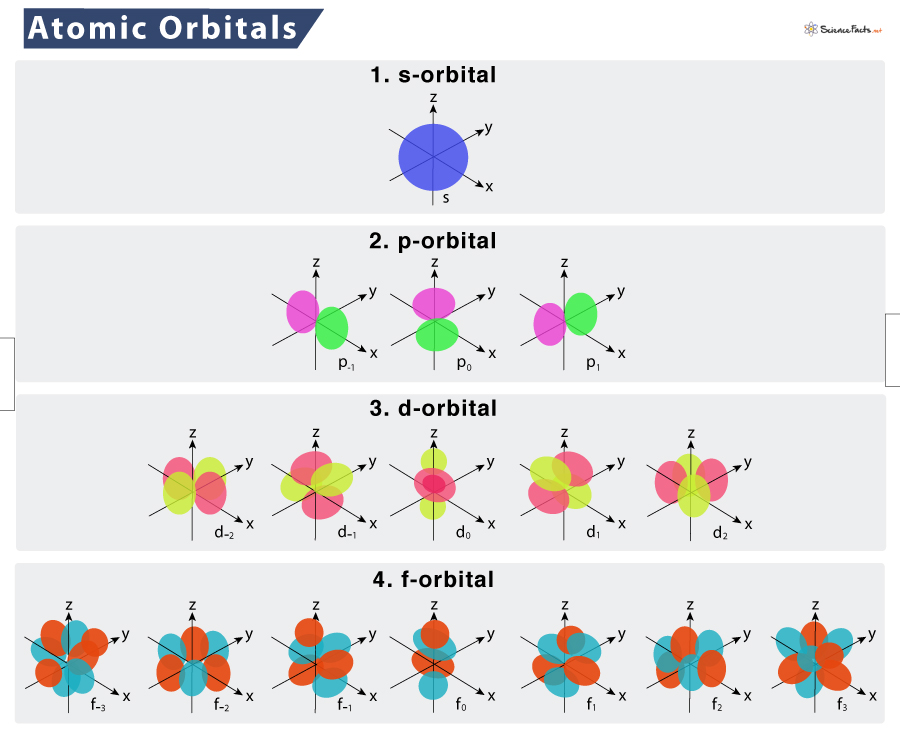
An electron orbitals magnetic quantum number ml is its orientation in space and it contains integral values in the range ll and the orbitals angular quantum number is l that defines its shape. The single s-orbitals where l0. Principal quantum number n Bangular momentum quantum number l C.
Magnetic Spin Quantum Number ms c. In atomic theory and quantum mechanics an atomic orbital is a mathematical function describing the location and wave-like behavior of an electron in an atom. Size of atomic orbital.
The spin quantum number ms. The angular momentum and magnetic quantum numbers together. E the angular momentum and magnetic quantum numbers together.
This makes the size of the atom roughly constant even as the number of electrons is heavier. The size of an atomic orbital is associated with A the principal quantum number n. D the spin quantum number ms.
The size of an atomic orbital is associated with The principal quantum number n The shape of an atomic orbital is associated with The angular momentum quantum number l The orientation in space of an atomic orbital is associated with the magnetic quantum number ml Atomic orbitals developed using quantum mechanics. The size of an atomic orbital is associated with. Magnetic Moment Quantum Number m d.
The size of the orbital is governed and decided by the principal quantum number n which is dependent on the overall average distance between the number of electrons as well as the nucleus. D the spin quantum number ms. The size of an atomic orbital is associated with A the principal quantum number n.

1 A The Atomic Orbitals S And P Of Carbon And B The Molecular S Download Scientific Diagram
0 Comments